
Who we are
We are scientists working at the interface of neuroscience and neurotechnology to understand the brain and its involvement in disease
We decipher disease and drug mechanisms and develop translational tools and therapeutics for mapping and modulating the brain

Michael Michaelides, Ph.D. Senior Investigator
Section Chief

Juan L. Gomez, Ph.D.
Staff Scientist
2015-present

Oscar Solís, Ph.D.
Research Fellow
2019-present

Marjorie Levinstein, Ph.D.
IRTA Postdoctoral Fellow
2021-present

Zachary Frangos, Ph.D. IRTA Postdoctoral Fellow
2023-present

Ingrid Schoenborn, B.S. IRTA Postbaccalaureate Fellow
2024-present

Anna Tischer, B.S.
IRTA Postbaccalaureate Fellow
2024-present

Will Dunne, B.S.
IRTA Postbaccalaureate Fellow
2024-present
Research
Mechanistic Neuropharmacology & Drug Discovery
We employ a wide array of complementary in vitro and in vivo pharmacological approaches for drug discovery and for dissecting the mechanism of action of popular CNS medications and recreational drugs.
Novel opioids & non-opioid analgesics
We are working on discovery and preclinical development of selective mu opioid receptor agonists for treatment of pain with low abuse liability and adverse effect profiles.
Ketamine & enantiomers
Ketamine is a controlled substance, has abuse potential, and can induce undesirable side effects. Nevertheless, it is considered to be generally safe and is a widely-used dissociative anesthetic and rapid-acting pain medication. The recent discovery that a single subanesthetic dose of racemic ketamine produces rapid and long-lasting antidepressant effects has been hailed as a key psychiatric breakthrough. (S)-ketamine (esketamine, SpravatoTM) was recently approved by the FDA as an intranasal formulation for treatment-resistant depression and human trials assessing efficacy of (R)-ketamine in depression are currently underway. As depression shares strong comorbidity with substance use disorders, we are working to better understand the precise in vitro and in vivo pharmacological properties and the abuse liability of its enantiomers.
(2R,6R)-Hydroxynorketamine
(2R,6R)-hydroxynorketamine (HNK) is a ketamine metabolite implicated in ketamine's efficacy in preclinical models of depression. We are working on the pharmacological characterization of (2R,6R)-HNK, for which human trials are underway.
Oliceridine (TRV-130)
Oliceridine is an FDA-approved pain medication. We are working on its in vivo pharmacological characterization and abuse liability profile.
Methadone & enantiomers
Racemic methadone is used for treatment of substance abuse. The efficacy of racemic methadone is attributed to (R)-methadone. (S)-methadone is being developed as a treatment for depression. We are working on the pharmacological characterization of (R)-methadone and (S)-methadone as well as their liability for abuse.

Gene-based neuromodulation: Chemogenetics
We develop translational genetic technologies for neuromodulation and non-invasive longitudinal imaging.
Precision medicine offers considerable advantages over conventional medical treatment. Within precision medicine, theranostics comprises a strategy that combines THERApeutic and diagNOSTIC strategies to provide a personalized treatment approach encompassing disease diagnosis, drug delivery, and disease/therapy monitoring using a single agent. Such interventions are timely given recent developments in neuromodulatory technologies. One such technology, called chemogenetics, offers the unprecedented ability to control neuronal activity in a cell type-specific manner without the need for chronically-implantable devices. A key feature of chemogenetic technologies is that they can be combined with clinical molecular imaging diagnostic methods such as positron emission tomography (PET). This combination extends the therapeutic component of chemogenetics to encompass its use in precision medicine-based neurotheranostics.
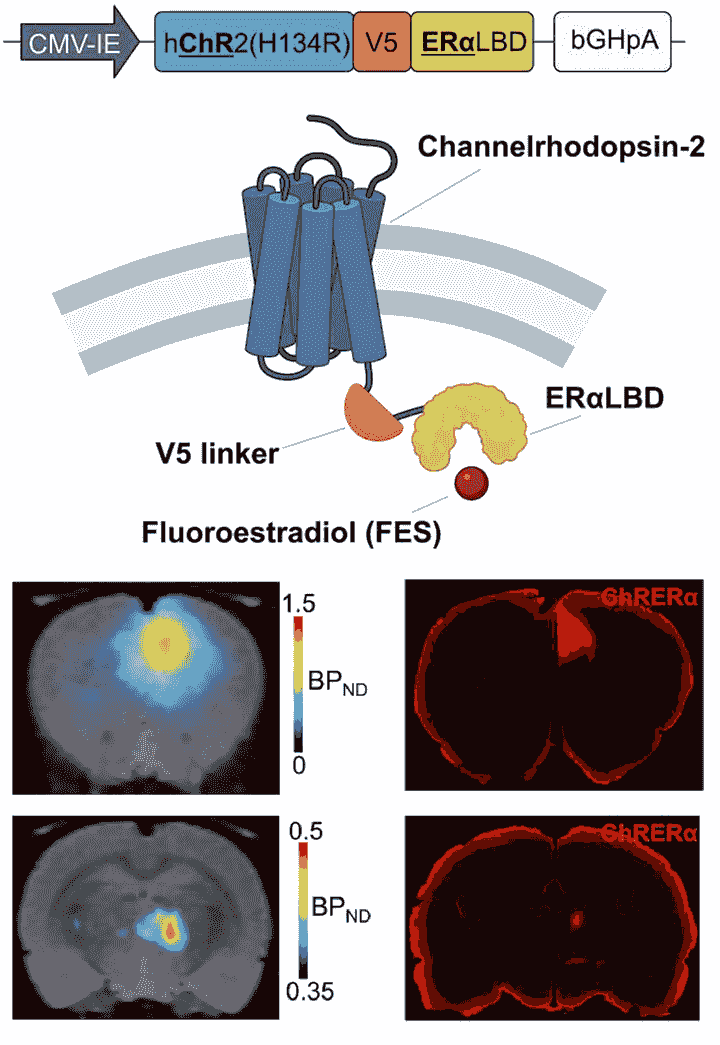
Gene-based neuromodulation: Optogenetics
Optogenetics is a widely-used technology consisting of light-activatable ion channels expressed in neural tissue which upon light stimulation can either activate or inhibit neurons with exquisite temporal precision. Optogenetics has had a strong impact on basic neuroscience research. However, its use in translational applications has been limited. One reason for this, is that to date, opsins have not been able to be visualized noninvasively in intact subjects. To address this, we are developing a noninvasive reporter detection system for optogenetics. This scalable system consists of (i) chimeric opsins tagged with a small human protein epitope and (ii) an FDA-approved PET radioligand and permits both optogenetic neuromodulation and noninvasive, quantitative, and longitudinal detection of opsins in the brain.
Figure: ChRERa (pronounced "carrera") consists of Channelrhodopsin-2 and the ligand binding domain (LBD) of human estrogen receptor alpha (ERa). It can be detected non invasively at the site of AAV-mediated transduction in the cortex and at corticothalamic terminal projection sites using PET and verified ex vivo using immunohistochemistry, allowing for non-invasive neuronal circuit tracing.
For more details our "Hot Topics" presentation at the ACNP 2019 meeting can be found here

Molecular imaging & PET radiotracer development
We perform PET studies by employing a variety of radioligands depending on experimental need. We perform noninvasive, quantitative, and longitudinal assessments of brain metabolic activity, neuroinflammation, neurotransmitter displacement, and receptor occupancy/target engagement of candidate compounds, genetic constructs, or other processes. We co-implement chemogenetic, optogenetic, pharmacological, focused ultrasound, or electrical stimulation with PET imaging in awake, freely-moving animals either in an exploratory fashion, to determine whole-brain functional networks recruited during behaviorally-relevant contexts, or to corroborate connectivity or target engagement of a defined neuron-type, region, or pathway. We use such approaches to map functional anatomy related to a variety of cell-types/projections in distinct brain regions in basic and translational research.
Radioligands we use:
[18F]FDOPA - Fluorinated L-Dopa for imaging dopamine terminals and levels
[18F]SynVest-1 - SV2A ligand - synaptic density marker
[18F]FDG - Glucose analog used for brain metabolic mapping
[11C]Raclopride - Displaceable dopamine D2/D3 receptor antagonist
[18F]Fallypride - Displaceable dopamine D2/D3 receptor antagonist
[18F]FE-DPN - Displaceable mu opioid receptor antagonist
[18F]FES - Estradiol analog for estrogen receptor and opsin imaging
[18F]JHU37107 - DREADD agonist
[11C]clozapine - atypical antipsychotic and DREADD agonist
[18F]ASEM - Nicotinic alpha7 receptor antagonist and PSAM4 agonist
Figure: PET image coregistered to MRI showing non-invasive assessment of dopamine D2/D3 receptors using [11C]raclopride in mouse brain.
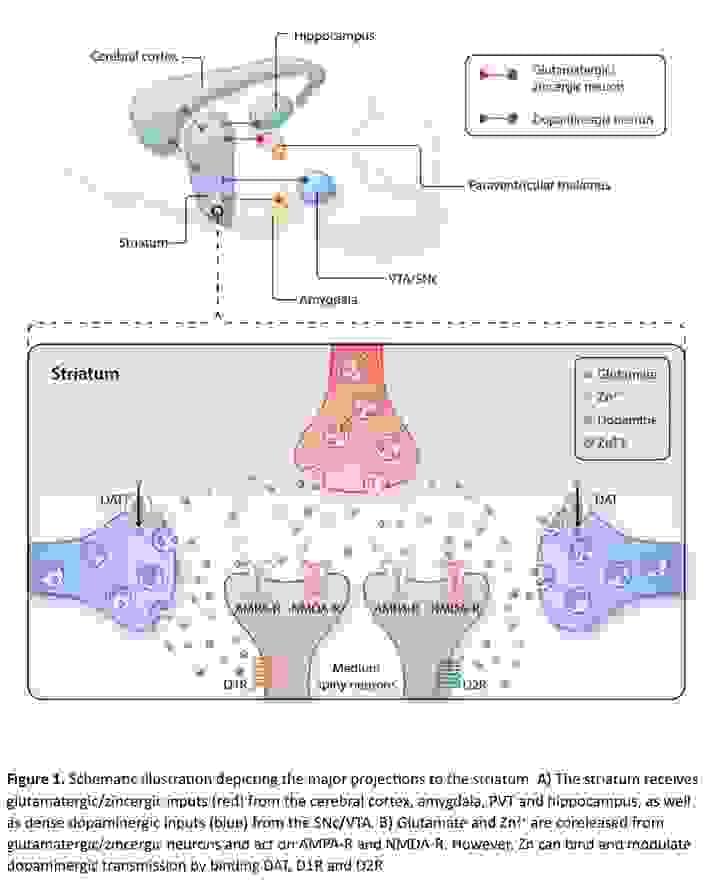
Synaptic Zinc
Synaptic zinc is essential for normal neurobiological functioning. Synaptic zinc and glutamate are transported into synaptic vesicles via the ZnT3 (Slc30a3) transporter and co-released from presynaptic terminals. People with addiction and related brain disorders have abnormal zinc levels. Nevertheless, the precise involvement of synaptic zinc in normal neurobiology or disease is not well understood. We are working to define the role of synaptic zinc in modulating neurochemistry and behavior and the extent to which this is relevant to addiction and related disorders.
Efforts involve development of ZnT3-cre mice and rats, dissecting the role of ZnT3-expressing neurons in behaviors relevant to addiction, and development of ZnT3-selective small molecules as tools and potential therapeutics.
Selected Publications

A Reversible Allosteric Inhibitor of GlyT2 Alleviates Neuropathic Pain Without On-target Side Effects
2025

Redefining Ketamine Pharmacology for Antidepressant Action: Synergistic NMDA and Opioid Receptor Interactions?
2025

Unique Pharmacodynamic Properties and Low Abuse Liability of the µ-Opioid Receptor Ligand
(S)-Methadone.2023
All Publications

A Reversible Allosteric Inhibitor of GlyT2 Alleviates Neuropathic Pain Without On-target Side Effects
2025

The Dopaminergic Effects of Esketamine are Mediated by a Dual Mechanism Involving Glutamate and Opioid Receptors
2025

Redefining Ketamine Pharmacology for Antidepressant Action: Synergistic NMDA and Opioid Receptor Interactions?
2025

Repetitive Grooming Behavior Following Aversive Stimulus Coincides with a Decrease in
Anterior Hypothalamic Area Activity2025

Sex Dependence of Opioid-mediated Responses to Subanesthetic Ketamine in Rats
2024

Unique Pharmacodynamic Properties and Low Abuse Liability of the µ-Opioid Receptor Ligand
(S)-Methadone.2023

DREADD-mediated Amygdala Activation is Sufficient to Induce Anxiety-like
Responses in Young Nonhuman Primates
2023

A Non-Canonical Striatopallidal “Go” Pathway that Supports Motor Control
2023
Tools & Resources

Radioligands & Imaging Agents
Our lab has pioneered the use of the agents below for non-invasive localization of chemogenetic and optogenetic switches in various species with the ultimate goal being human application.
[3H]ASEM - In vitro PSAM4-GlyR & PSAM4-5HT3 quantification
[18F]ASEM - Longitudinal In vivo PSAM4-GlyR & PSAM4-5HT3 quantification
[3H]Clozapine - In vitro hM3Dq/hM4Di quantification
[3H]Compound 13 (C13) - In vitro hM3Dq/hM4Di quantification
[18F]JHU37107 (J07) - Longitudinal In vivo hM3Dq/hM4Di quantification
[18F]fluoroestradiol (FES) - Longitudinal In vivo Opsin quantification (coming soon)
Publications

Chemogenetic agonists
Our lab has developed the first DREADD agonists that exhibit high affinity, high in vivo potency, and high brain penetrance in several species, which favors clinical translation. These compounds require very low systemic doses (<0.1 mg/kg) to facilitate rapid and remote activation of chemogenetic switches in the brain.

Plasmids
For translational and clinical gene therapy applications, chemogenetic/optogenetic switches need to be optimized to drive efficient expression and trafficking to the cell membrane, where their respective actuators would achieve maximum efficacy. For this reason, our goal has been to optimize existing chemogenetic/optogenetic gene therapy constructs for optimal expression and targeting. One way of doing this is to strip bulky and potentially toxic fluorescent reporters, typically used in such designs, and which are not useful for translational and clinical applications. Our strategy is to leverage the use of our translational PET-based reporters for non-invasive and longitudinal quantification of chemogenetic/optogenetic switches along with small epitopes (e.g. HA-tag) whenever in vitro detection would be necessary.
Highlights

Nature: Research Briefing on our recent article
Our recent work highlighted in the journal Nature
September 3 2025

The role of the estrogen receptor in COVID-19
Our recent work highlighted in the journal Nature Italy. Interview by first author Dr. Oscar Solis.
December 12 2022

Cooperative Research and Development Agreement (CRADA) with Attune Neurosciences, Inc.
Mike Michaelides to serve as a co-PI on a CRADA with Attune Neurosciences, Inc. to develop focused ultrasound applications for neuromodulation
December 22 2021

Cooperative Research and Development Agreement (CRADA) with Redpin Therapeutics, Inc.
Mike Michaelides to serve as a PI on a CRADA with Redpin Therapeutics, Inc. to develop chemogenetic applications for translational applications
June 16 2021

NIH Scientists Redesign Neurons to Enable Targeted Therapies
Our recent work highlighted in the NIH I am Intramural Blog
March 17 2021


Presentation at the Brain Initiative's "Chemogenetic Innovations in the Manipulation & Monitoring of Labeled Neurons Workshop"
Mike presented at this NIH Brain Initiative Workshop
December 10 2019

Presentation at the ACNP 2019 Annual Meeting describing our optogenetics molecular imaging technology
Mike presented at the ACNP 2019 "Hot Topics"
December 10 2019

"Changing the Locks" article and interview for Chemistry World about our work and that of others on chemogenetics.
Mike's interview at Chemistry World
May 20 2019

Interview for Science magazine: Could deep brain stimulation help zap diabetes?
Mike's interview for Science
May 23 2018

Interview for the American Psychiatric Association (APA): DREADDs Could Guide More Targeted Treatments in Future
Mike's interview for Psychiatric News
March 16 2018

Research highlight about our recent work on chemogenetics
Our recent work highlighted in the journal Nature Methods
September 29 2017

Research highlight about our recent work on chemogenetics
Our recent work highlighted in the journal Nature Chemical Biology
September 19 2017
Alumni

Fallon Curry, B.S.
IRTA Postbaccalaureate Fellow
2022-2024
Current - Ph.D. student
University of Minnesota

Reece Budinich, B.S.
IRTA Postbaccalaureate Fellow
2021-2024
Current - Ph.D. student
University of Pittsburgh

Leila Ghaffari, B.S.
Special Volunteer
2021-2024
Current - Clinical Research Coordinator
University of Pennsylvania

Emilya Ventriglia, B.S., M.S.
IRTA Postbaccalaureate Fellow
2020-2023
Current - Ph.D. student
Brown Universtiy-NIH GPP Program

Matthew Boehm, Ph.D.
PhD Student, NIH GPP Program/Brown University
2017-2022
Current - AAAS Fellow
Department of Veterans Affairs

Meghan Carlton, B.S.
IRTA Postbaccalaureate Fellow
2019-2021
Current - PhD student
Albert Einstein School of Medicine

Jordi Bonaventura, Ph.D.
Research Fellow
2019-2021
IRTA Postdoctoral Fellow
2016-2019
Current - Ramón y Cajal Investigator
University of Barcelona

Sherry Lam, B.S.
Research Technician
2019-2020
IRTA Postbaccalaureate Fellow
2017-2019
Current - M.A. student
Rutgers University

Theresa Kopajtic, B.S.
Research Biologist
2018-2019
Current - Retired

Kelsey Wright, B.S.
IRTA Postbaccalaureate Fellow
2017-2019
Current - Ph.D. Student
Northwestern University

Dondre Marable, B.S.
IRTA Postbaccalaureate Fellow
2017-2019
Current - Product Manager, CTM

Jatia Mills, B.S.
RTURP Research Fellow
Summer 2018
Current - Ph.D. student
Biomedical and Veterinary Sciences, Virginia Tech

Weilin Chan, B.S.
Special Volunteer/Summer Student
2016-2018
Current - M.D. Student
University of Buffalo

Randall J. Ellis, B.S.
IRTA Postbaccalaureate Fellow
2015-2017
Current - Ph.D. student
Biophysics & Systems Pharmacology
Icahn School of Medicine at Mount Sinai

Lionel A. Rodriguez, B.S.
IRTA Postbaccalaureate Fellow
2015-2017
Current - Ph.D. student,
Neuroscience
Johns Hopkins University

Margaret Jokoh
RTURP Research Fellow
Summer 2016
Current - Student, Loyola University

Kat Daly, B.S.
Lab rotation, NIH GPP program
Spring 2016
Current - Ph.D. student
JHU/NIH GPP Program
BIMN Lab © 2017





















































































































